| Definitions and criteria of anaphylaxis |
| Year |
Source |
Definitions and statements |
Diagnostic criteria |
| 1945 |
Cooke RA. Allergy in theory and practice. Philadelphia, PA: W. B. Saunders Company; 1945:5. |
Defined anaphylaxis as “A special of particular immunologic type of induced protein (or hapten) sensitivity in man or experimental animals and may properly be considered as a subdivision of allergy.” |
|
| 1970s |
Nonspecific; in text of book “Anaphylaxis and hypersensitivity reactions” edited by M Castells |
Defined anaphylaxis as “a systemic, immediate hypersensitivity reaction caused by IgE-mediated immunologic release of mediators from mast cells and basophils.”
Defined anaphylactoid reaction as “a similar reaction without evidence of IgE involvement.”
|
|
| 1998 |
Joint Task Force on Practice Parameters |
Defined anaphylaxis as “immediate systemic reaction caused by rapid, IgE-mediated immune release of potent mediators from tissue mast cells and peripheral basophils.”
Defined anaphylactoid reaction as “reaction that mimic signs and symptoms of anaphylaxis, but are caused by a non-IgE mediated release of potent mediators from mast cells and basophils.” |
|
| 2003 |
World Allergy Organization (WAO) |
Expanded definition of anaphylaxis to include immunologic events (with or without IgE) and non-immunologic (contrast, vibration, temperature, etc).
Recommended term “anaphylactoid reaction” be abandoned and term “nonallergic anaphylaxis” used instead. |
|
| 2004 |
Brown, SGA. Clinical features and severity grading of anaphylaxis. Journal of Allergy and Clinical Immunology 2004: 114(2), 371-376. |
Recommended grading system for generalized hypersensitivity reactions. |
Grade 1: Mild
Skin and subcutaneous tissues only |
Generalized erythema (redness/flushing), urticaria (hives), periorbital edema (swelling around eyes), or angioedema (swelling).
Can also be subdivided as with or without angioedema. |
| Grade 2: Moderate
Features suggesting respiratory, cardiovascular, or gastrointestinal involvement |
Dyspnea (difficulty breathing), stridor, wheeze, nausea, vomiting, presyncope (dizziness/about to pass out), diaphoresis (sweating), chest or throat tightness, or abdominal pain. |
| Grade 3: Severe
Hypoxia (low blood oxygenation), hypotension (low blood pressure), or neurologic compromise |
Cyanosis (turning blue), pulse oxygenation less than 92%, systolic blood pressure below 90 mm Hg (for adults), confusion, collapse, loss of consciousness, incontinence |
| 2005 |
Sampson HA, et al. Symposium on the definition and management of anaphylaxis: summary report. J Allergy Clin Immunol 2005; 115(3), 584-591. |
Recommended three specific scenarios that would identify anaphylaxis. Cautioned that these criteria are for classic anaphylaxis and may not cover non-immunologic anaphylaxis cases, such as exercise anaphylaxis.
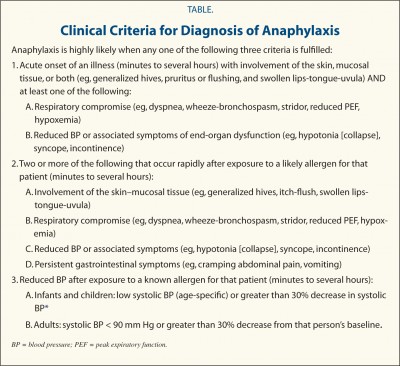
Anaphylaxis is likely when any 1 of the 3 criteria are fulfilled. |
1 |
Onset of illness within minutes to hours with involvement of:
Skin/mucosal tissue (hives, generalized itch, flush, swollen tips/tongue) and airway compromise (difficulty breathing, wheezing, bronchospasm, stridor, reduced peak expiratory flow) |
| Onset of illness within minutes to hours with involvement of:
Skin/mucosal tissue (hives, generalized itch, flush, swollen tips/tongue) and low blood pressure or associated symptoms (low muscle tone, fainting) |
| 2 |
Onset of illness within minutes to hours with involvement of two or more of the following after exposure to known allergen for that patient:
History of severe allergic reaction
Skin/mucosal tissue (hives, generalized itch, flush, swollen tips/tongue)
Airway compromise (difficulty breathing, wheezing, bronchospasm, stridor, reduced peak expiratory flow)
Low blood pressure or associated symptoms (low muscle tone, fainting)
In suspected food allergy: gastrointestinal symptoms (crampy abdominal pain, vomiting) |
| 3 |
Onset of low blood pressure within minutes to hours after exposure to known allergen for that patient:
For adults, systolic blood pressure less than 100 mm Hg or decrease from baseline by 30% or more
For children, low systolic blood pressure for age or decrease from baseline by 30% or more
|
| 2006 |
Sampson HA, et al. Second symposium on the definition and management of anaphylaxis: summary report – Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol 2006: 117(2), 391-397. |
Recommended three specific scenarios that would identify anaphylaxis. Cautioned that these criteria may only identify 95% of anaphylaxis cases.
Anaphylaxis is highly likely when any 1 of the 3 criteria are fulfilled.
These definitions have been used frequently to identify whether or not epinephrine is indicated to treat potential anaphylaxis.
These criteria have been validated, please refer to:
Campbell RL, et al. Evaluation of National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network criteria for the diagnosis of anaphylaxis in emergency department patients. J Allergy Clin Immunol 2012; 129, 748-752. |
1 |
Onset of illness within minutes to several hours with involvement of:
Skin/mucosal tissue (generalized hives, itching, flushing, swollen lips/tongue/uvula) and airway compromise (difficulty breathing, wheezing, bronchospasm, stridor, reduced peak expiratory flow, low blood oxygenation)
Onset of illness within minutes to several hours with involvement of:
Skin/mucosal tissue (generalized hives, itching, flushing, swollen lips/tongue/uvula) and low blood pressure or associated symptoms of end-organ dysfunction (low muscle tone/collapse, fainting, incontinence) |
|
| 2 |
Onset of illness within minutes to several hours with involvement of two or more of the following after exposure to likely allergen for that patient:
History of severe allergic reaction
Skin/mucosal tissue (generalized hives, itching, flushing, swollen lips/tongue/uvula)
Airway compromise (difficulty breathing, wheezing, bronchospasm, stridor, reduced peak expiratory flow, low blood oxygenation)
Low blood pressure or associated symptoms of end-organ dysfunction (low muscle tone/collapse, fainting, incontinence)
Persistent gastrointestinal symptoms (crampy abdominal pain, vomiting) |
|
3 |
Onset of low blood pressure within minutes to hours after exposure to known allergen for that patient:
For adults, systolic blood pressure less than 100 mm Hg or decrease from baseline by 30% or more
For children, low systolic blood pressure for age or decrease from baseline by 30% or more |
| 2007 |
Kroigaard M, et al. Scandinavian clinical practice guidelines on the diagnosis, management and follow-up of anaphylaxis during anesthesia. Acta Anaesthesiol Scand 2007: 51, 655-670. |
Assign severity grades for anaphylaxis. |
Grade 1 |
Generalized skin symptoms: flushing, hives, possible angioedema |
| Grade 2 |
Moderate multiorgan involvement with skin symptoms, low blood pressure, tachycardia, airway reactivity (cough, difficulty breathing) |
| Grade 3 |
Severe life threatening multiorgan involvement that requires specific treatment: collapse, tachycardia or bradycardia, arrhythmias, bronchospasm. Skin symptoms may not be present. |
| Grade 4 |
Circulatory or respiratory arrest |
| Grade 5 |
Death due to a lack of response to cardiorespiratory resuscitation |
| 2007 |
Ruggeberg JU, et al. Anaphylaxis: Case definition and guidelines for data collection, anaphylaxis, and presentation of immunization safety data. Vaccine 2007: 25, 5675-5684. |
Both defined anaphylaxis and attached diagnostic algorithm for “level of diagnostic certainty”, a measure of how confident a provider could be that anaphylaxis was the correct diagnosis.
Anaphylaxis was defined as “a clinical syndrome characterized by sudden onset AND rapid progression of signs and symptoms AND involving multiple (2 or more) organ systems as follows.” |
Level 1 of diagnostic certainty
Highest level of certainty |
One or more major dermatological symptom (generalized hives or flushing, local or generalized angioedema, generalized itching with rash) and one or more major cardiovascular symptom (low blood pressure, shock with at least 3 of 4 manifestations: tachycardia, capillary refill time >3 seconds, reduced central pulse volume, decreased level of consciousness or loss of consciousness) |
|
|
One or more major dermatological symptom (generalized hives or flushing, local or generalized angioedema, generalized itching with rash) and one or more major respiratory symptom (bilateral wheeze, stridor, upper airway swelling, respiratory disease with 2 or more of the following: rapid respiration, increased use of accessory respiratory muscles, recession, cyanosis, grunting) |
| Level 2 of diagnostic certainty
Moderate level of certainty |
One or more major cardiovascular symptom (low blood pressure, shock with at least 3 of 4 manifestations: tachycardia, capillary refill time >3 seconds, reduced central pulse volume, decreased level of consciousness or loss of consciousness) and one or more major respiratory symptom (bilateral wheeze, stridor, upper airway swelling, respiratory disease with 2 or more of the following: rapid respiration, increased use of accessory respiratory muscles, recession, cyanosis, grunting) |
| One or more major cardiovascular symptom (low blood pressure, shock with at least 3 of 4 manifestations: tachycardia, capillary refill time >3 seconds, reduced central pulse volume, decreased level of consciousness or loss of consciousness) and one or more minor criterion involving one or more system besides cardiovascular or respiratory (generalized itching without rash, generalized prickle sensation, localized injection site urticaria, red and itchy eyes, diarrhea, abdominal pain, nausea, vomiting, mast cell tryptase elevation above normal limit)
|
| One or more major respiratory symptom (bilateral wheeze, stridor, upper airway swelling, respiratory disease with 2 or more of the following: rapid respiration, increased use of accessory respiratory muscles, recession, cyanosis, grunting) and one or more minor criterion involving one or more system besides cardiovascular or respiratory (generalized itching without rash, generalized prickle sensation, localized injection site urticaria, red and itchy eyes, diarrhea, abdominal pain, nausea, vomiting, mast cell tryptase elevation above normal limit) |
| One or more major cardiovascular symptom (low blood pressure, shock with at least 3 of 4 manifestations: tachycardia, capillary refill time >3 seconds, reduced central pulse volume, decreased level of consciousness or loss of consciousness) and one or more major dermatological symptom (generalized hives or flushing, local or generalized angioedema, generalized itching with rash) and one or more minor cardiovascular symptom (reduced peripheral circulation with at least 2 of 3 manifestations: tachycardia, capillary refill time >3 seconds without low blood pressure, decreased level of consciousness) |
| One or more major cardiovascular symptom (low blood pressure, shock with at least 3 of 4 manifestations: tachycardia, capillary refill time >3 seconds, reduced central pulse volume, decreased level of consciousness or loss of consciousness) and one or more major dermatological symptom (generalized hives or flushing, local or generalized angioedema, generalized itching with rash) and one or more minor respiratory symptom (persistent dry cough, hoarse voice, difficulty breathing without wheeze or stridor, sensation of throat closure, sneezing, stuffy nose)
|
| One or more major respiratory symptom (bilateral wheeze, stridor, upper airway swelling, respiratory disease with 2 or more of the following: rapid respiration, increased use of accessory respiratory muscles, recession, cyanosis, grunting) and one or more major dermatological symptom (hives or flushing, local or generalized) angioedema, generalized itching with rash) and one or more minor cardiovascular symptom (reduced peripheral circulation with at least 2 of 3 manifestations: tachycardia, capillary refill time >3 seconds without low blood pressure, decreased level of consciousness) |
| One or more major respiratory symptom (bilateral wheeze, stridor, upper airway swelling, respiratory disease with 2 or more of the following: rapid respiration, increased use of accessory respiratory muscles, recession, cyanosis, grunting) and one or more major dermatological symptom (generalized hives or flushing, local or generalized angioedema, generalized itching with rash) one or more minor respiratory symptom (persistent dry cough, hoarse voice, difficulty breathing without wheeze or stridor, sensation of throat closure, sneezing, stuffy nose) |
| Level 3 of diagnostic certainty
Low level of certainty |
One or more minor respiratory symptom (persistent dry cough, hoarse voice, difficulty breathing without wheeze or stridor, sensation of throat closure, sneezing, stuffy nose) and one or more minor criterion involving two or more systems besides cardiovascular or respiratory (generalized itching without rash, generalized prickle sensation, localized injection site urticaria, red and itchy eyes, diarrhea, abdominal pain, nausea, vomiting, mast cell tryptase elevation above normal limit)
|
| One or more minor cardiovascular symptom (reduced peripheral circulation with at least 2 of 3 manifestations: tachycardia, capillary refill time >3 seconds without low blood pressure, decreased level of consciousness) and one or more minor criterion involving two or more systems besides cardiovascular or respiratory (generalized itching without rash, generalized prickle sensation, localized injection site urticaria, red and itchy eyes, diarrhea, abdominal pain, nausea, vomiting, mast cell tryptase elevation above normal limit) |
| 2010 |
Ring J, et al. History and classification of Anaphylaxis. Chem Immunol Allergy 2010: 95, 1-11. |
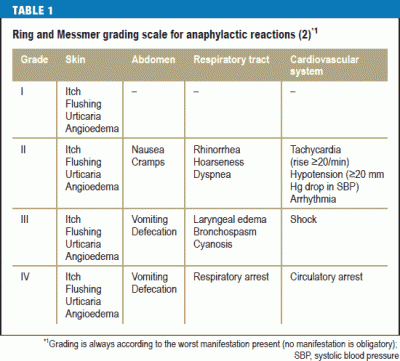
Assigned severity grade based upon worst symptom |
Grade 1 |
Skin (itching, flushing, urticaria, angioedema) |
| Grade 2 |
Skin (itching, flushing, urticaria, angioedema may or may not be present
GI (nausea, cramps)
Respiratory (stuffy nose, hoarseness, difficulty breathing, arrhythmia) Cardiovascular (increase of over 20 bpm, systolic blood pressure decreased by at least 20 mm Hg) |
| Grade 3 |
Skin (itching, flushing, urticaria, angioedema may or may not be present)
GI (vomiting, defecation)
Respiratory (swelling in airway, bronchospasm, turning blue)
Cardiovascular (shock) |
| Grade 4 |
Skin (itching, flushing, urticaria, angioedema may or may not be present)
GI (vomiting, defection)
Respiratory (respiratory arrest)
Cardiovascular (circulatory arrest) |
| 2013 |
Ito K. Diagnosis of food allergies: the impact of oral food challenge testing. Asia Pac Allergy 2013: 3(1): 59-69. |
Identified severity of anaphylaxis based upon grade of most severe symptom. |
Grade 1
Per primary source, grade 1 is NOT considered anaphylaxis. |
Skin (local itching, rash, hives, angioedema)
GI (oral itchiness, discomfort, lip swelling)
Respiratory (throat itchiness, discomfort) |
| Grade 2 |
Skin (systemic itching, rash, hives, angioedema)
GI (nausea, vomiting, diarrhea, transient colic)
Respiratory (mild nasal congestion, sneezing, single coughing)
Neurologic (loss of activity) |
| Grade 3 |
Skin (systemic itching, rash, hives, angioedema)
GI (repeated vomiting, diarrhea, persistent colic)
Respiratory (severe nasal congestion, repeated sneezing, continuous coughing, throat itching)
Cardiovascular (heart rate increased by 15 bpm or more)
Neurologic (anxiety) |
| Grade 4 |
Skin (systemic itching, rash, hives, angioedema)
GI (repeated vomiting, diarrhea, persistent colic)
Respiratory (choking sensation, hoarse voice, barking cough, difficulty in swallowing, wheezing, trouble breathing, turning blue)
Cardiovascular (arrhythmia, decreased blood pressure)
Neurologic (irritability, sense of impending doom) |
| Grade 5 |
Skin (systemic itching, rash, hives, angioedema)
GI (repeated vomiting, diarrhea, persistent colic)
Respiratory (respiratory arrest)
Cardiovascular (severe bradycardia, severe hypotension, cardiac arrest)
Neurologic (loss of consciousness) |